Passing ISO 13485 and CE Marking audits is a critical milestone for medical device manufacturers. These audits are not just about meeting compliance requirements, they’re about proving that your products are safe, effective, and ready for the market. In this guide, we’ll walk you through what to expect from these audits, how they’re connected, and provide tips to help you prepare for success.
What is ISO 13485 and CE Marking?
ISO 13485 is the international standard for quality management systems (QMS) in the medical device industry. It ensures that medical devices are consistently designed, manufactured, and delivered to meet regulatory requirements, maintaining quality throughout the product lifecycle.
CE Marking is the symbol that signifies a product’s compliance with EU regulations and allows it to be sold in Europe under the EU Medical Device Regulation (MDR) 2017/745. CE Marking confirms that your device meets the EU’s strict safety and performance standards.
How Do ISO 13485 and CE Marking Work Together?
ISO 13485 and CE Marking are closely linked. To successfully meet CE Marking requirements, your QMS must align with ISO 13485. Here’s how the two work together:
- ISO 13485 as your foundation for MDR compliance: Your QMS needs to align with ISO 13485 to demonstrate compliance with the EU MDR.
- Risk Management: Both ISO 13485 and CE Marking focus heavily on identifying, mitigating, and managing risks throughout the product lifecycle.
Post-Market Surveillance and Traceability: Both require systems for tracking product performance and addressing issues once the device is on the market.
ISO 13485: A Comprehensive Overview
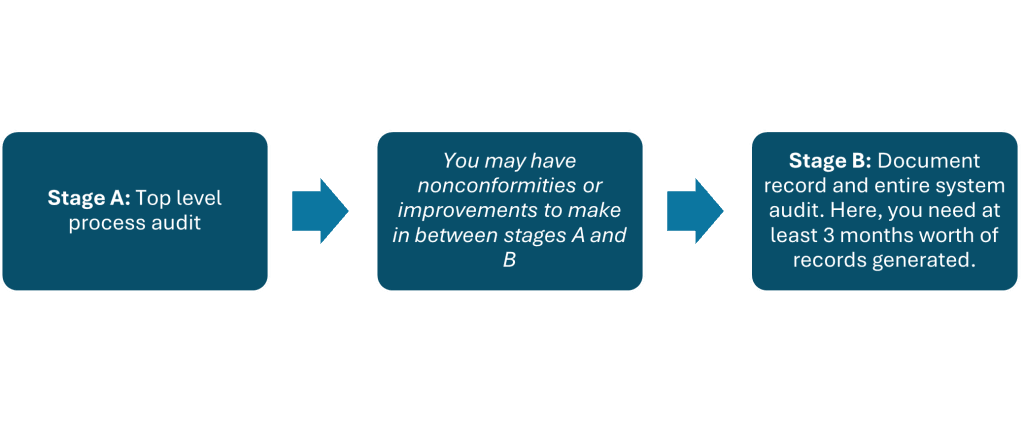
An ISO 13485 audit evaluates how well your QMS supports the safe design, production, and delivery of medical devices. This audit typically occurs in two stages:
- Stage 1: Initial audit focusing on your company’s QMS documentation.
- Stage 2: A more thorough audit of your QMS in action, including an on-site inspection and assessment of manufacturing processes, controls, and compliance with regulatory standards.
The ISO 13485 audit helps ensure your QMS adheres to the necessary standards for medical device production, which is essential for CE Marking.
How to Prepare for ISO 13485 & CE Marking Audits
Preparation is key to passing ISO 13485 and CE Marking audits. Here are some practical tips to help you get ready:
1. Build a Solid Quality Management System (QMS)
Your QMS should be clear, structured, and easy to navigate. Auditors will check if your QMS aligns with ISO 13485 and supports regulatory compliance. Key actions include:
- Ensure processes are documented and easily accessible.
- Embed the QMS throughout the entire organisation and business operations.
2. Risk Management & Mitigation Strategies
ISO 13485 requires a risk-based approach to managing medical device risks, as outlined in ISO 14971. Be prepared to:
- Show how you identify, assess, and control risks at every stage of the product lifecycle.
- Maintain thorough documentation of risk management activities and decisions.
3. Design and Development Process Considerations
Auditors expect clear traceability throughout the design and development process. You’ll need to:
- Provide detailed records of user needs, design inputs, design outputs, and validation.
- Keep a record of design reviews, design changes, and approval processes.
4. Supplier Management
Your supplier management process must be robust, transparent, and compliant. Make sure you:
- Have a system in place to select, evaluate, and monitor suppliers.
- Keep audit records and evidence of ongoing supplier reviews.
- Perform regular risk assessments on your suppliers.
5. Training & Competency
Proper training is essential for audit success. Ensure that all team members are properly trained and competent in their roles. To meet this requirement:
- Maintain a comprehensive training matrix.
- Document competency assessments and continuous training efforts.
Post-Audit: What Happens After ISO 13485 & CE Marking Audits?
After the audit, the auditor will provide a report detailing any non-conformities or areas for improvement. Be prepared to address these issues promptly. This feedback is a valuable opportunity to enhance your QMS and ensure long-term compliance.

Preparing for Both ISO 13485 and CE Marking Audits
Although ISO 13485 and CE Marking audits may seem daunting, with the right preparation, you can approach them with confidence. Here’s a checklist to help you prepare:
- Ensure your QMS is fully aligned with ISO 13485 and EU MDR.
- Have a clear risk management strategy in place that aligns with ISO 14971.
- Organise and maintain your technical documentation for easy access during the audit.
- Keep track of design and development processes with clear records of validation and approvals.
- Document ongoing post-market surveillance and feedback loops.
How LFH Regulatory Can Help You Succeed
Navigating ISO 13485 and CE Marking audits can be complex, but with expert guidance, you can streamline the process. Here’s how LFH Regulatory can assist:
- ISO 13485 & CE Marking Audit Preparation
- QMS Implementation & Support
- Risk Management Strategies
- Clinical Evaluation and Documentation
- Supplier Management
- Ongoing Consultancy Support
- Regulatory Intelligence and Notified Body Support
Contact us today to ensure your medical devices are ready for market and compliant with regulatory requirements.
Need Further Help?
If you have any questions or need assistance with ISO 13485 and CE Marking audits, get in touch with our expert consultants today. We offer comprehensive support to guide you through the process.
Call us on +441484662575 or email us at info@lfhregulatory.co.uk.
Final Thoughts
With proper preparation and expert guidance, passing your ISO 13485 and CE Marking audits can be straightforward. Focus on building a solid QMS, managing risks effectively, and ensuring complete traceability throughout the product lifecycle. Let LFH Regulatory be your partner in navigating the regulatory landscape, ensuring that your products are compliant and ready for market.
FAQ’s for Succeeding in ISO 13485 and CE Marking
What is the purpose of an ISO 13485 audit?
An ISO 13485 audit evaluates whether your Quality Management System (QMS) meets international standards for medical device design, manufacture, and delivery.
Why is CE Marking important for medical devices?
CE Marking confirms that your medical device complies with EU MDR requirements, proving it meets strict safety and performance standards for sale in Europe.
How are ISO 13485 and CE Marking connected?
To achieve CE Marking, your QMS must align with ISO 13485. This ensures your processes support MDR compliance, risk management, and post-market surveillance.
What happens during an ISO 13485 audit?
The audit is usually conducted in two stages:
Stage 1 reviews QMS documentation.
Stage 2 assesses your QMS in practice, including on-site inspections of processes, records, and compliance.
How should companies prepare for these audits?
Key steps include:
Building a structured, compliant QMS
Documenting risk management in line with ISO 14971
Maintaining traceability in design and development
Implementing robust supplier management
Ensuring staff training and competence
What is expected after the audit?
Auditors provide a report with any non-conformities or recommendations. Companies must correct issues promptly to maintain compliance and improve their QMS.
Do smaller manufacturers need to comply with ISO 13485 and CE Marking?
Yes. Regardless of size, all medical device manufacturers must demonstrate compliance with ISO 13485 and CE Marking requirements to sell in Europe.
How can external consultants help with audit preparation?
Consultants can guide you through QMS implementation, technical documentation, risk management, and liaising with Notified Bodies to streamline the audit process.
Zara Malik
Zara works closely with a wide range of clients, supporting them and the wider team at LFH
in bringing medical devices and in vitro diagnostics (IVDs) to market. Her role spans internal
operations and project management, responding to a variety of client queries on quality and
regulatory matters, supporting the development of Quality Management Systems, Technical
Documentation, and assisting with Risk Management activities; ensuring compliance
throughout the product lifecycle.
With over 10 years of experience in the industry, Zara began her career in the laboratory of
an IVD company, where she quickly developed an interest in regulatory affairs. She went on
to specialise in risk management and internal auditing at a large medical device
organisation, before expanding her expertise into Technical Documentation and Post-Market
Surveillance during the implementation of the EU MDR and IVDR. Zara then joined a start-
up, gaining hands-on experience with Software as a Medical Device (SaMD) and AI/ML-
based medical technologies. She now brings this broad and evolving expertise to LFH
Regulatory, supporting clients across a range of complex and emerging regulatory
challenges.




