Creating a usability file is essential in the product development process for medical devices, including Software as a Medical Device (SaMD), to ensure user-centred design and safety. IEC 62366-1 is the global standard outlining the process for usability testing in medical devices. This comprehensive guide will explore the critical steps involved in creating a usability file that meets IEC 62366-1 requirements, from defining user needs to post-market surveillance.
What is IEC 62366-1?
IEC 62366-1 is an international standard that defines the usability testing process for medical devices. This standard emphasises understanding user needs, tasks, and environments to reduce the risks associated with user errors and enhance the effectiveness of medical devices.
A usability file is a documented record of the usability engineering process throughout the lifecycle of a device. It is crucial for demonstrating compliance with regulatory requirements and ensuring both the safety and effectiveness of the device from the user’s perspective.
Key Components of an IEC 62366-1 Usability File for Medical Devices
To ensure compliance with IEC 62366-1, creating a comprehensive usability file is a vital part of the design process. Here are the key steps involved:
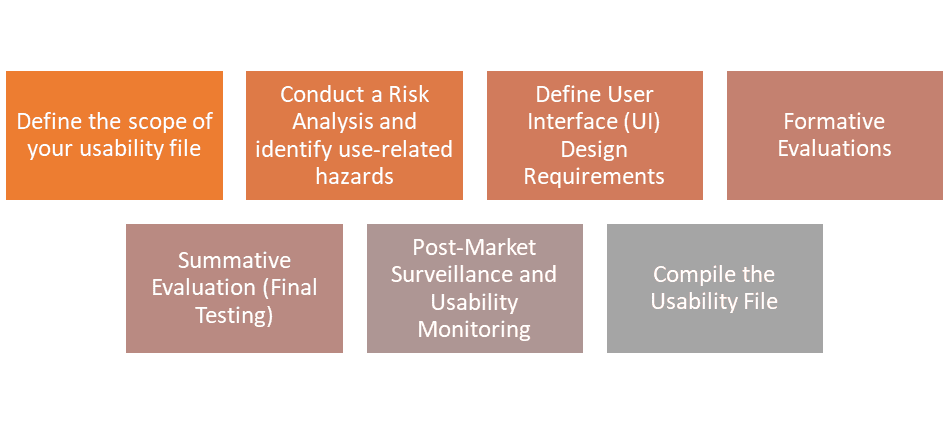
1. Define the Scope of Your Usability File
The first step in the usability engineering process is to clearly define the scope of the usability file. This includes the intended users, the intended use, and the environment in which the device will be used.
- Intended Users: Identify who will use the device. This could include healthcare professionals, patients, caregivers, or other stakeholders.
- Intended Use: Clarify what the device is meant to do, whether it’s for monitoring, diagnosing, or therapeutic purposes.
- Use Environment: Consider where the device will be used. Different environments (e.g., home use vs. hospital settings) can impact usability significantly.
Tip: Include a clear description of these elements in your file. They are typically summarised in a ‘use specification document’ that forms the foundation of your usability engineering process.
2. Conducting a Risk Analysis for Usability Testing in Medical Devices
IEC 62366-1 requires conducting a thorough risk analysis to identify potential hazards that could arise due to user errors or poor design. Here’s how you can approach it:
- Hazard Identification: Identify potential hazards related to user errors or inadequate device design. For example, could a patient misinterpret a warning or misuse a medical app?
- Risk Assessment: Evaluate the likelihood and severity of harm caused by each identified hazard. Consider whether a user action could lead to patient harm, incorrect diagnosis, or delayed treatment.
Tip: Document each identified hazard and its associated risk in a hazard analysis table. Also, define the mitigation strategies for each risk.
3. Defining User Interface (UI) Design Requirements for Medical Devices
After understanding user needs and conducting risk analysis, define UI design requirements that meet the needs of your intended user population. This step is critical to ensure usability and safety.
- Human Factors: Consider cognitive, physical, and sensory capabilities of your users. For instance, if the device is intended for elderly patients, make sure the fonts are large, and the interface is simple and easy to navigate.
- Design Specifications: Include clarity in labelling, controls, alarms, and instructions for use (IFU). Adapt these elements for specific groups such as non-professional users or those with visual impairments.
- Task Flow: Minimise errors by designing an interface where critical tasks are easy to access and perform without unnecessary steps.
Tip: Include UI specifications and design rationales in your usability file, explaining how the interface reduces errors and improves usability.
4. Formative Evaluations: Early Prototyping and Testing
Formative evaluations are a vital part of the usability engineering process. These tests involve evaluating early prototypes with users to identify potential problems before final design completion.
- User Testing: Conduct usability tests with representative users to evaluate the device’s interface and interaction design. This can include cognitive walkthroughs, task analysis, and heuristic evaluations.
- Iterative Testing: Based on feedback, iterate on the design to address issues found during testing. This can involve revising the UI, adjusting instructions, or simplifying tasks.
Tip: Document the test plans, protocols, and results from formative evaluations. Also, include user feedback and any design changes made based on the results.
5. Summative Evaluation: Final Usability Testing
Summative evaluation is the final stage of usability testing. It focuses on validating the final design to ensure it meets usability goals and performs safely and effectively in real-world scenarios.
- Final Usability Testing: Conduct usability testing on the final device to confirm that it performs as expected and users can operate it safely. This often involves more realistic use scenarios, possibly in clinical or home settings.
- Task Performance Metrics: Measure whether users can successfully complete key tasks, such as using the device without errors, interpreting information correctly, and following safety instructions.
Tip: Summarise the results of the summative evaluation, including task completion rates, time on task, error rates, and any corrective actions taken based on the findings.
6. Post-Market Surveillance and Usability Monitoring
After the product has been launched, ongoing usability monitoring is essential. Post-market surveillance helps identify emerging usability issues and areas for improvement.
- Incident Reporting: Establish a system for collecting feedback and reports on user-related issues, including complaints, adverse events, or usability failures.
- Usability Improvements: If issues are identified post-market, address them through iterative design improvements or updates.
Tip: Include a plan for post-market usability monitoring in your usability file. This plan should detail how to collect feedback and address any usability concerns after the device is launched.
7. Compile the Usability File
The final usability file should provide a comprehensive record of the entire usability engineering process, from risk analysis and design specifications to testing and post-market surveillance. This file demonstrates that all necessary steps have been followed to ensure the device is safe and effective.
Tip: Organise the usability file into clear sections that document each stage of the process, including:
- Scope and intended use of the device
- Hazard analysis and risk assessment
- Design specifications and UI guidelines
- Formative and summative test results
- Post-market usability monitoring plan
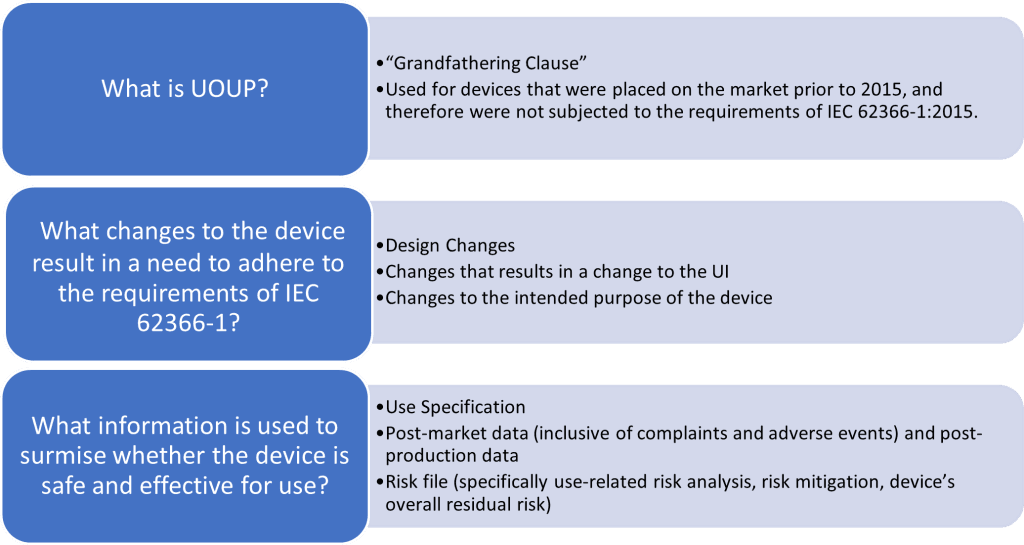
What About Devices Placed on the Market Before IEC 62366-1:2015?
For devices that were launched before the IEC 62366-1:2015 standard, there’s a special clause in the standard called ‘User Interface of Unknown Provenance (UOUP)’. This provides guidelines on how such devices can still be made compliant with the requirements.
Final Thoughts
Writing a usability file according to IEC 62366-1 is critical for ensuring the safety, effectiveness, and user-friendliness of your medical device or SaMD. By following a structured usability engineering process—starting from defining user needs, conducting risk analyses, to iterative testing and post-market surveillance—you’ll be able to create a comprehensive file that meets regulatory requirements and enhances the user experience.
Usability is an ongoing process. Regular testing, feedback, and updates are essential to maintaining safety and usability throughout the device’s lifecycle. A well-maintained usability file will not only provide vital evidence for regulatory submissions but also contribute to continuous improvement and product safety.
Need Expert Help with IEC 62366-1 Usability Testing?
If you have any questions or need assistance with your usability file creation, get in touch with our regulatory specialists today! We can guide you through the process and ensure your medical device is fully compliant with IEC 62366-1.
Contact us:
📧 Email: info@lfhregulatory.co.uk
📞 Phone: +44 (0) 1484 662 575
FAQs:
What is IEC 62366-1?
IEC 62366-1 is an international standard for usability testing in medical devices, ensuring that the device is designed to minimise user errors and improve safety and effectiveness.
How do I create a usability file according to IEC 62366-1?
A usability file includes documentation of user needs, risk analyses, UI design requirements, formative and summative testing, and post-market surveillance to comply with IEC 62366-1.