Spotlight on Anisha Rose Chacko, Clinical Consultant

We’re delighted to welcome Anisha Rose Chacko to the LFH Regulatory team as our new Clinical Consultant. We sat down with Anisha to learn more about her background, what inspired her move to LFH, and what she’s most looking forward to in her new role. Can you tell us a little about your background and […]
Spotlight on Morgan Malone, Business Development Manager

We’re delighted to welcome Morgan Malone to the LFH Regulatory team as our new Business Development Manager. We sat down with Morgan to learn more about her background, what inspired her move to LFH, and what she’s most looking forward to in her new role.
LFH on the Road – Meet the Team at Upcoming Events
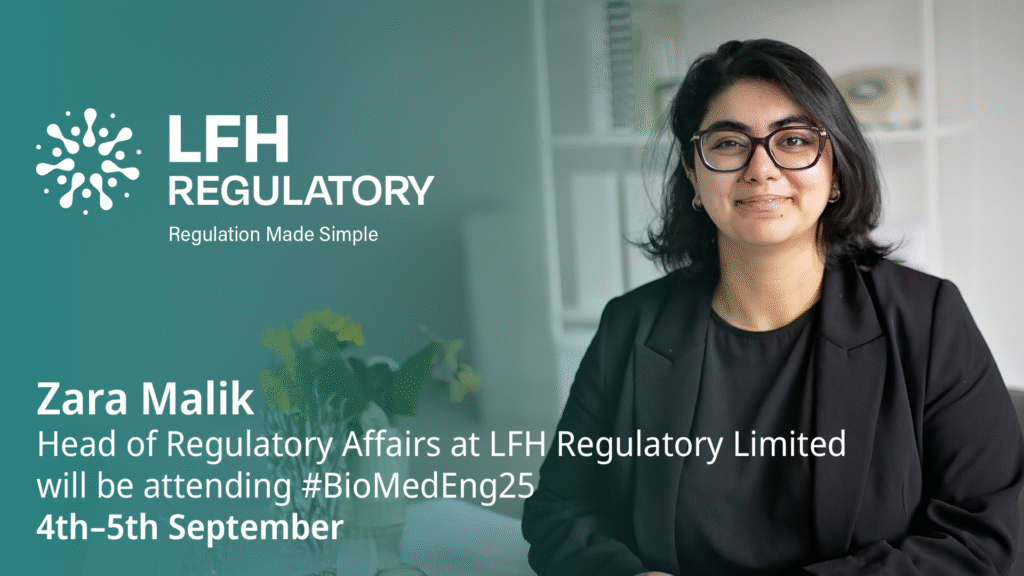
The LFH Regulatory team will be out and about over the coming months, attending a range of leading industry events across the UK and internationally. These events provide a fantastic opportunity to connect with our experts, discuss the latest regulatory challenges, and explore how we can support your business in navigating complex UK, EU and […]
LFH Regulatory launches ISO 13485 QMS template packages

For medical device organisations at every stage – from start-up to scale-up LFH Regulatory has launched a new service: ISO 13485 QMS Template Packages, created to make Quality Management System (QMS) implementation faster, easier, and more cost-effective for medical device organisations. For companies that design, develop, produce, install, or service medical devices, a QMS aligned […]
Welcoming Our New Starters to LFH Regulatory

We’re thrilled to introduce three fantastic new team members who bring a unique blend of expertise, energy, and passion for health tech innovation. Chloe Johnson – Junior QA/RA ConsultantWhat attracted you to join LFH Regulatory? I was drawn to LFH Regulatory by its dynamic involvement with leading manufacturers at the forefront of health tech innovation. […]
LFH Regulatory Supports MedTech 2025 ‘Pitch Your Product’ Competition

LFH Regulatory was proud to support the Pitch Your Product: MedTech 2025 event hosted at Pennine HQ in collaboration with Health Innovation East Midlands. This high-energy event saw 18 innovative companies each deliver a two-minute pitch to a panel of expert judges. All were competing for a prize package worth £20,000 – designed to help […]
