The Growing Importance of Regulatory Intelligence
With regulatory requirements continuously evolving and the increasing stringency of medical device legislation, it’s no surprise that regulatory compliance can feel overwhelming, especially for manufacturers managing multiple markets. While Regulatory Intelligence (RI) may appear simple for low-risk devices such as Class I non-sterile products, the process becomes significantly more complex for Class III implantable devices with integrated software applications. Using a well-structured Regulatory Intelligence strategy not only supports regulatory compliance, but also helps to reduce time to market, minimise costs, and avoid delays due to non-conformance.
What is Regulatory Intelligence?
Regulatory Intelligence is defined as:
A systematic process of collecting, analysing, and disseminating information about regulatory requirements, policies, and guidelines that impact the development, manufacturing, distribution, surveillance, and approval of medical devices.
This information spans global regulations, standards, guidance documents, updates, and market-specific requirements.
Regulatory professionals—especially in small or medium-sized enterprises—are often tasked with covering an immense amount of territory with limited resources. This makes an effective RI strategy not just helpful but critical to success.
The Value of Regulatory Intelligence
A robust Regulatory Intelligence process helps manufacturers to:
- Monitor and anticipate changes in regulatory requirements
- Ensure market readiness and ongoing compliance
- Prioritise resources effectively
- Reduce risk of non-compliance
- Build proactive strategies for product registration, clinical evaluation, and post-market surveillance
Monitoring Changes in Medical Device Regulation
Staying informed is the first and most critical step. Regulatory Intelligence starts with a comprehensive understanding of the current global regulatory landscape, followed by continuous tracking for upcoming changes and developments that may impact device compliance.
Key Sources for RI May Include:
- Regulatory authority websites (e.g. MHRA, FDA, EMA)
- Standards organisations (e.g. ISO, IEC)
- Industry associations
- Guidance documents (e.g. MDCG guidance)
- Public consultations and press releases
- Clinical research databases
- Subscription-based regulatory databases and newsletters
What Are the Main Stages of Regulatory Intelligence Activities?
To implement an effective RI process, manufacturers should follow these key stages:
- Identification – Determine which regulations, markets, and standards are relevant.
- Collection – Gather information from validated sources.
- Assessment – Review and interpret how the information affects your devices.
- Dissemination – Share findings with key stakeholders (e.g. RA/QA, R&D, clinical teams).
- Action – Implement changes or updates to documentation, procedures, or strategy.
Maintenance – Regularly update your RI system to ensure continued compliance.

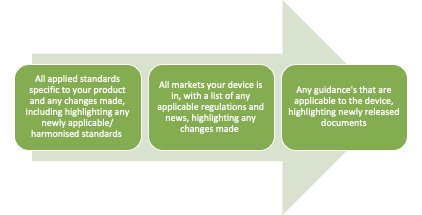
What Information Should Be Included?
Your Regulatory Intelligence documentation should include:
- Regulatory updates (EU, UK, US, global)
- New and revised standards
- Guidance documents
- Safety alerts and recalls
- Post-market surveillance trends
- Labelling and packaging changes
- Emerging technology trends and their regulatory implications
Challenges in Building a Strong Regulatory Intelligence Framework
Despite its value, many organisations face common challenges when trying to establish a comprehensive RI system:
- Difficulty accessing or interpreting emerging market regulations
- Language barriers with regional legislation
- Spending excessive time on research with limited output
- Poor coordination between departments, leading to fragmented information
- Lack of structured data, leading to underutilised intelligence
- Absence of a centralised, accessible RI repository
Final Thoughts…
In today’s complex regulatory environment, Regulatory Intelligence is not a luxury—it’s a necessity. It enables manufacturers to stay ahead of changes, remain compliant, and make informed decisions that ensure patient safety and product success.
Having the right tools, expertise, and team in place can dramatically improve how regulatory information is collected, used, and shared across the business.
Still Unsure What to Do? LFH Regulatory Can Help…
At LFH Regulatory, we understand the challenges medical device manufacturers face in gathering, evaluating, and acting on regulatory intelligence. Whether you’re developing a new device or managing a global portfolio, our experienced consultants can support you in:
- Creating a Regulatory Intelligence strategy
- Conducting global regulatory scans
- Reviewing product compliance and technical documentation
- Navigating new market regulations
📞 Call us: +44 1484 662575
📧 Email us: info@lfhregulatory.co.ukOr simply complete the ‘Contact Us’ form to get in touch.
FAQ’s for Regulatory Intelligence in Medical Devices
What is Regulatory Intelligence in medical devices?
Regulatory Intelligence (RI) is the systematic process of collecting, analysing, and applying information about regulatory requirements, standards, and guidance that affect the development, approval, and monitoring of medical devices.
Why is Regulatory Intelligence important?
It helps manufacturers stay compliant with evolving regulations, avoid costly delays, reduce risks of non-conformance, and speed up time to market.
What are the key stages of a Regulatory Intelligence process?
The main stages are:
Identification of relevant regulations and markets
Collection of validated information
Assessment of impact on devices
Dissemination of findings to stakeholders
Action through updates and strategy changes
Maintenance of a living RI system
What information should be tracked in Regulatory Intelligence?
Manufacturers should monitor regulatory updates, new and revised standards, guidance documents, safety alerts, recalls, post-market surveillance trends, and regulatory implications of emerging technologies.
What are the biggest challenges in Regulatory Intelligence?
Common issues include interpreting new market regulations, language barriers, fragmented information across teams, time-consuming research, and lack of a centralised RI repository.
How does Regulatory Intelligence support compliance with EU MDR and IVDR?
RI helps manufacturers track new guidance, standards, and notified body expectations, ensuring technical documentation, clinical evaluation, and post-market surveillance stay aligned with regulatory requirements.
How often should Regulatory Intelligence be updated?
It should be updated continuously as new regulations, standards, and guidance are released. A robust RI process ensures changes are acted upon quickly.
What tools are used for Regulatory Intelligence?
Sources include regulatory authority websites (e.g. MHRA, FDA, EMA), ISO/IEC standards bodies, MDCG guidance, industry associations, clinical databases, and subscription-based RI platforms.
Who in the organisation should use Regulatory Intelligence?
RI should be shared with regulatory affairs, quality assurance, R&D, and clinical teams to ensure decisions are consistent and compliant across all departments.
Can small and medium-sized manufacturers manage Regulatory Intelligence effectively?
Yes, but they often benefit from expert consultancy support to create a structured RI strategy, centralise updates, and avoid resource-intensive manual tracking.



