Knowing the regulation is only half the battle. Understanding how to apply those regulations to your devices in a smart and innovative way, whilst ensuring compliance, can make all the difference. A good regulatory strategy doesn’t just focus on compliance, but it also develops the base for the design and development through to commercialisation and regulatory submissions of your device.
Why do regulatory strategies matter?
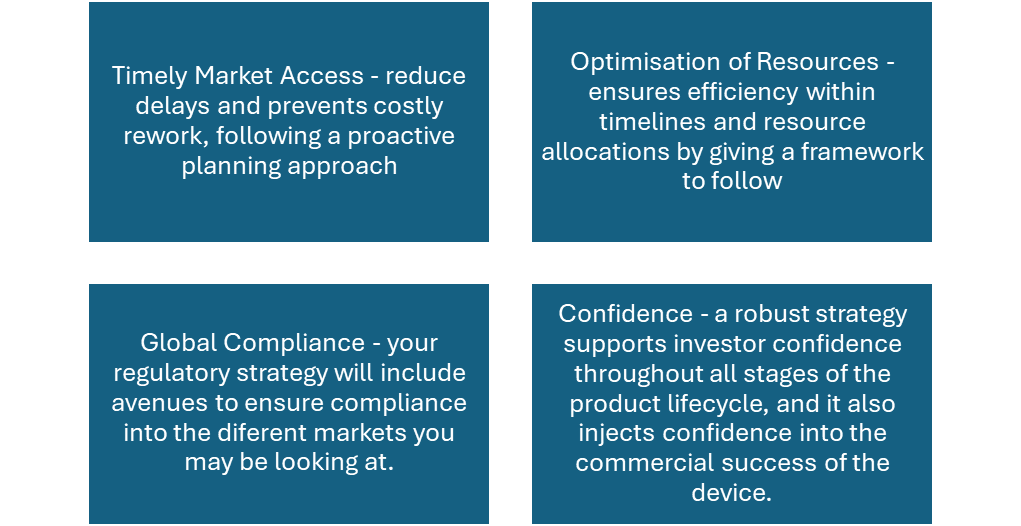
When should you compile your strategy?
Early planning of your regulatory strategy helps to understand which route to market will work best for your device – for example, if you are planning to release within the UK or EU, early planning will help you to understand whether to go straight for a CE marking strategy, or whether to go for a UKCA compliance mark first and a CE mark down the line (depending on the classification of the device in both markets).
A strategy ideally should be done within the design review stage, before design freeze, to understand how to strategically apply the regulations to your product, whilst ensuring compliance. This will also help with understanding basic timelines.
What are the key aspects of a regulatory strategy?
- Device classification:
- Understand which risk class the device falls under. If you are planning to launch in the UK, EU and/or US, it helps to perform a medical device classification against each of the regions, this way, a decision can be made on which route to market to take first.
- It is important to take into account, not only what the device is stated as doing in its intended purpose, but how the device actually works.
- Intended Use and Population
- Define specific medical purpose, any indications/contra-indications, target users, target demographic and the use environment.
- This will also be useful in determining the device classification.
- Approval pathway
- Assess and provide different pathways, with an strategic conclusion on which pathway would be the best option, based on risk classification, novelty of the device, and taking into account any regions where an audit may be needed.
- Evidence strategy
- List necessary testing, clinical and non-clinical requirements. This can include biocompatibility, clinical evidence planning, clinical trials, usability, electrical safety testing where necessary.
Applying Regulations in a Smart Way
A good regulatory strategy should be well-researched to help combat potential hurdles en route to market.
It’s important to get buy in for the regulatory strategy from early, as we’ve discussed above, the earlier the better for a regulatory strategy. It’s important not to view regulations as a ‘hurdle’ to overcome, when in reality they provide frameworks that can help you better plan for getting your product to market. For higher-risk devices, which require Notified Body involvement and meeting strict Notified Body requirements, we would recommend engaging with a Notified Body earl, to establish clear timelines and lines of communication. For example, if you have received information from a Notified Body, that there is a 6-month lead time between application and stage 1 audit, these 6 months can be used to finalise documentation. This is more efficient than having all documentation ready for audit, then engaging with a Notified Body and having to wait an additional 6 months when you’re ready for audit now.
As was mentioned above, it’s important that your strategy looks at global compliance, in all regions. It’s important to take into account the classification of the device in all proposed marketing regions, this will allow you to assess the routes to market in different regions, and align on a pathway going forward that results in the route of least resistance whilst being compliant, and ensuring your device performs as you have intended. You should also note that having a robust regulatory intelligence system can help with monitoring and anticipating regulatory shifts. This is helpful as it allows you to adapt strategies proactively, to ensure that you are always compliant. It is also a helpful tool to help you stay in line with and regulatory alignments that have occurred (for example,part of an effective FDA medical device strategy is understanding alignment of US 21CFR820 with ISO 13485).
Conclusion
An effective regulatory strategy doesn’t just take into account manufacturers and potential investors, but consumers too. It is your pathway to getting your device approved, sold and used, safely and legally. Plan early, be accurate and intentional with your intended purpose, think strategically with your classification, take into account each regions requirements, and understand which testing needs to be carried out for global market access.
Need help with your regulatory strategy? Don’t hesitate to get in touch with us. We’re always happy to help.
Frequently Asked Questions About Regulatory Strategies for Medical Devices
What is a regulatory strategy for medical devices?
A regulatory strategy is a structured plan that outlines how a medical device will meet compliance requirements across different markets. It considers classification, intended use, approval pathways, and evidence requirements to ensure a smooth route to market.
When should I develop a regulatory strategy?
The best time is during the design review stage, before design freeze. Early planning helps you align classification, timelines, and regulatory pathways, making compliance smoother and reducing costly delays later in development.
Why is device classification important in a regulatory strategy?
Classification defines the level of risk associated with your device and determines which regulatory pathway applies. It varies between the UK, EU, and US, so assessing classification in each region ensures you choose the most efficient route to market.
How does intended use affect a regulatory strategy?
Intended use shapes your classification and evidence requirements. Clearly defining purpose, user population, and environment of use ensures your strategy is accurate, defensible, and aligned with compliance expectations.
What is an evidence strategy in medical device regulation?
An evidence strategy sets out all required clinical and non-clinical testing, such as biocompatibility, usability, electrical safety, or clinical trials. It ensures you collect the right data at the right time to support regulatory submissions.
Should I engage with a Notified Body early?
Yes. For higher-risk devices requiring Notified Body involvement, early engagement helps establish timelines, avoid long waiting periods, and align expectations on documentation and audits.
How can I build a global regulatory strategy?
Consider each region’s classification and approval requirements, look for regulatory alignments such as FDA 21CFR820 with ISO 13485, and plan pathways that minimise duplication while maintaining compliance across markets.
What role does regulatory intelligence play in strategy?
A strong regulatory intelligence system helps you monitor changes, anticipate new requirements, and adapt your strategy proactively. This avoids compliance gaps and supports faster, safer global market access.
Amrita Bansal
Amrita supports with Quality and Regulatory knowledge for medical devices throughout the
product lifecycle, helping to get innovative devices on the market for patients. She also helps
with overseeing the Regulatory Intelligence efforts, including impact assessment and global
regulatory surveillance.
Amrita has a background in product registrations, medical information, product technical
complaints for pharmaceuticals and combination products before transitioning to regulatory
affairs where she has continued to build up her expertise. Amrita has helped numerous
clients with different portions of their regulatory journeys, from a full QMS implementation, to
usability, to technical file creation, to regulatory strategy and classification.
Amrita is driven by a passion for patient safety, having seen first-hand real-world impacts of
bringing safe and reliable products to market. She combines her knowledge with her
practical expertise to ensure that she can deliver strategic but compliant approaches for her
clients and their medical devices/ technologies.




