Introduction to Regulatory Affairs and Change Management
Change is a constant in the medical device industry. From evolving regulations to technological advancements and shifts in product design, manufacturers must stay agile to thrive. Regulatory affairs play a critical role in managing these changes to ensure compliance with global standards, maintain product quality, and continue meeting market demands.
Navigating regulatory frameworks while adapting to change requires a structured and proactive approach. For medical device manufacturers, understanding how to manage change efficiently is crucial to staying competitive and compliant in an increasingly complex landscape.
Drivers of Change in the Medical Device Industry
The medical device industry is constantly evolving, and several factors contribute to the pace of change:
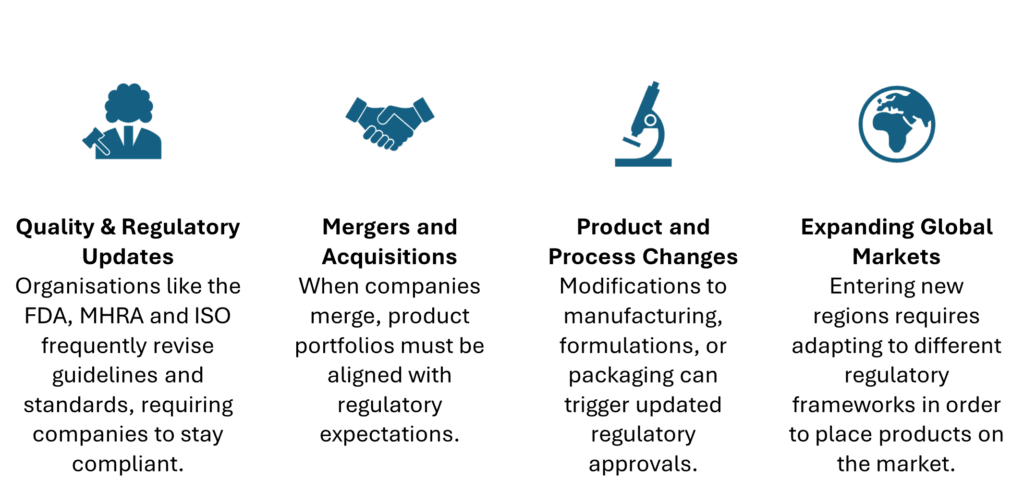
- Evolving Regulations: Regulatory standards are updated frequently to reflect new scientific advancements, patient safety concerns, or emerging technologies. Compliance with these standards is essential to avoid penalties and ensure continued access to markets.
- Technological Advancements: The integration of cutting-edge technology into medical devices requires frequent updates and modifications. Innovations in AI, machine learning, and data analytics, for example, bring new opportunities but also require strict adherence to regulatory requirements.
- Market Demands and Competitive Pressure: Manufacturers must adapt to consumer expectations and competitive forces. Regulatory changes or shifts in product design can help meet these demands while maintaining product quality and safety.
- Quality and Safety Standards: Continuous improvements in quality control and patient safety often drive regulatory changes. New guidelines and best practices ensure products meet the highest standards, benefiting both manufacturers and consumers.
Why Change Management is Important
Proper change management is essential for maintaining compliance and ensuring that medical device manufacturers stay competitive. Here are some key reasons why:
- Ensuring Compliance
Regulatory frameworks are continually evolving, and companies must stay ahead of these changes to avoid non-compliance, which can lead to fines, product recalls, or even loss of market access. An effective change management strategy ensures that regulatory changes are addressed quickly and efficiently. - Reducing Risk
Poorly managed changes can introduce risks to product quality, patient safety, and regulatory compliance. A structured approach helps mitigate these risks by ensuring that any changes made are properly evaluated and documented, preventing unintended consequences. - Enhancing Efficiency
A well-defined change management process helps prevent unnecessary delays, streamline approval processes, and improve coordination between departments. This increases overall efficiency, allowing manufacturers to respond more quickly to changes without disrupting their operations. - Maintaining Market Competitiveness
Manufacturers that adapt quickly to regulatory changes and industry trends maintain their market position and capitalise on new opportunities. Proactively managing changes allows companies to stay ahead of competitors who might struggle with adapting to new regulations.
Supporting Business Continuity
A robust change management process ensures that operations continue smoothly despite regulatory shifts or organisational changes. Having clear guidelines in place ensures that your company can navigate changes without disrupting business continuity.
Common Challenges in Change Management
Navigating regulatory change management in the medical device industry can be challenging. Common obstacles include:
- Regulatory Uncertainty
Frequent updates to standards and regulations across global markets can create complexities. Manufacturers must constantly stay informed of changes in local and international regulations to avoid falling out of compliance. - Resistance to Change
Employees may be reluctant to adopt new processes or ways of working that result from regulatory changes. Overcoming this resistance requires strong leadership and clear communication about the benefits and necessity of these changes. - Tight Deadlines
Compliance deadlines often require manufacturers to make quick adjustments within short timeframes. This pressure can lead to rushed decisions, which may result in compliance risks or operational errors. - Data Management Issues
Handling large volumes of documentation and ensuring that all records are accurate and up-to-date can be cumbersome without the right systems in place. Manual processes or outdated systems can slow down the change management process. - Communication Barriers
In larger organisations, it can be difficult to know who to communicate with when a change is being implemented. Clear communication strategies are vital to ensuring everyone involved is informed and aligned.
How to Manage Changes Effectively
Here are some best practices for managing regulatory changes in the medical device industry:
1. Have a Clear Change Control Strategy
A structured change control strategy is the foundation of effective change management. Key steps include:
- Establish a Change Control Board (CCB): A CCB, composed of representatives from different departments, provides input on potential changes and assesses their impact before implementation.
- Assign Clear Roles and Responsibilities: Assigning clear roles for managing and implementing changes ensures accountability and smooth execution of the process.
- Document All Changes: Proper documentation of all changes, along with their approvals, is essential for tracking and ensuring compliance.
2. Assess the Impact Thoroughly
Before implementing any change, consider the following questions:
- How will this change affect the safety and efficacy of the product?
- Is the change considered significant or substantial?
- Are there any compliance risks or regulatory implications from this change?
- What operational or supply chain impacts might arise?
- Will this change affect registrations in other markets?
This thorough evaluation will help identify any risks associated with the change and ensure that the decision is well-informed.
3. Engage Key Stakeholders
Collaboration between departments like regulatory affairs, R&D, quality assurance, and manufacturing is essential to ensuring that all aspects of a change are considered and that compliance is maintained. In a global environment, changes in one location can impact other parts of the supply chain, so international coordination may also be necessary.
4. Keep Documentation Up to Date
If it’s not written down, it didn’t happen! Maintaining accurate and transparent records helps ensure smooth audits, inspections, and internal traceability. All changes should be well-documented for compliance purposes.
5. Monitor and Review Changes
After implementing a change, continuous monitoring is necessary to ensure that the change remains compliant and is functioning as intended. Regular reviews allow for the identification of any potential areas for improvement.
Leveraging Technology in Change Management
Digital tools have revolutionised the way regulatory affairs teams manage change. Some key technologies include:
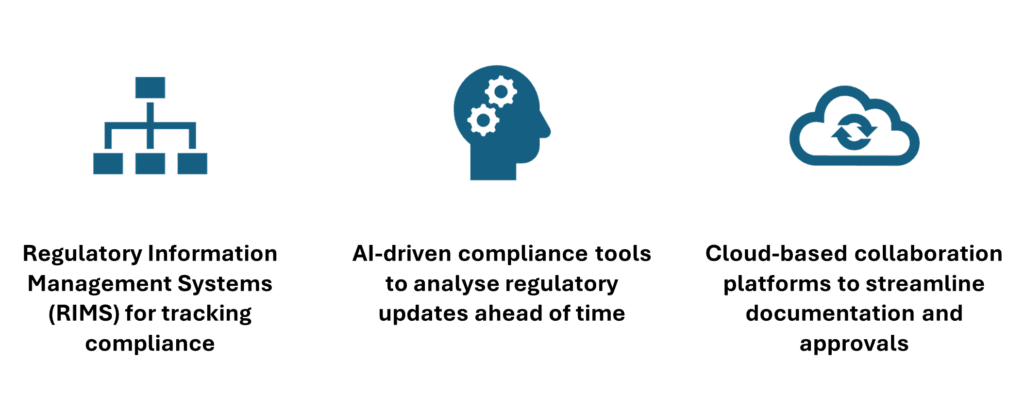
- Regulatory Information Management Systems (RIMS): These systems help track regulatory requirements and ensure that compliance documents are properly managed across all stages of the product lifecycle.
- AI-Driven Compliance Tools: Artificial intelligence tools can automate compliance tasks, helping to identify potential regulatory issues before they become a problem.
- Cloud-Based Collaboration Platforms: These platforms enable teams to collaborate in real-time, ensuring that all stakeholders are kept informed and that approvals can be managed quickly and efficiently.
By leveraging these digital solutions, manufacturers can significantly improve their change management processes and reduce compliance risks.
Final Thoughts
Change management doesn’t have to be overwhelming. By implementing a structured approach, conducting thorough impact assessments, engaging key stakeholders, and leveraging technology, medical device manufacturers can stay compliant while adapting to industry shifts. Mastering change management will help ensure long-term, sustainable success in an ever-evolving and fast-paced industry.
While it may seem daunting, with the right preparation, strategies, and expertise, you will be able to avoid the pitfalls that come with change and remain competitive in the marketplace.
How Can LFH Help?
At LFH, we understand the challenges of regulatory compliance and change management in the medical device industry. Our expert services include:
- Technical Documentation
- Risk Management
- Clinical Evaluation
- Usability Studies
- Biological Evaluation
- Intended Use Support
- Ongoing Consultancy Support
- QMS Implementation
- Audit Support
- Notified Body Support
We can make regulations easy for you and support you every step of the way. Contact us today to learn more about how we can assist with your change management needs.