Overview of the Clinical Evaluation for Medical Devices
CE Marking is essential for selling medical devices in Europe under the EU Medical Device Regulation (MDR) 2017/745. As outlined in Article 61 and Annex XIV of the EU MDR, manufacturers must plan, conduct, and document clinical evaluation reports. This evaluation ensures that medical devices and Software as a Medical Device (SaMD) meet safety and performance standards for regulatory compliance.
Why Do I Need a Clinical Evaluation for Medical Devices & SaMD?
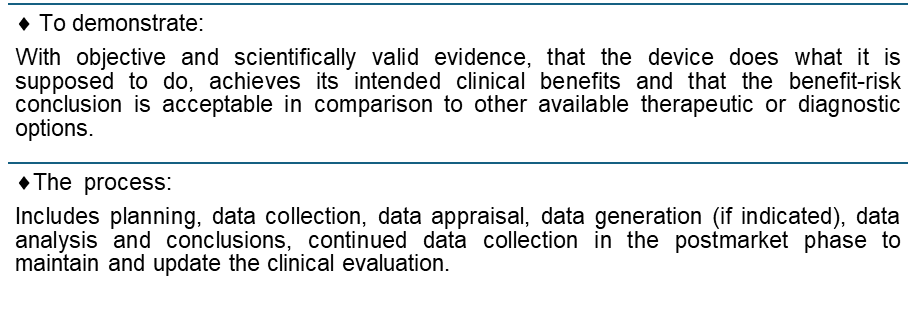
The Clinical Evaluation Report (CER) is one of the most critical documents in the development and regulation of medical devices. It demonstrates that your device is safe, effective, and meets its intended purpose. A well-crafted CER is an essential part of ensuring your device meets EU MDR requirements and can be legally sold in the European market.
In this blog, we will walk you through the essential steps for creating effective clinical evaluation reports for medical devices and SaMD, and provide tips for compliance with EU MDR.
Key concepts for creating an effective Clinical Evaluation Report
A Clinical Evaluation Report (CER) is a detailed analysis of both pre-market and post-market clinical data that are relevant to your medical device or SaMD. The CER must cover both technical and clinical aspects of the device, ensuring it is suitable for its intended use.
Key Elements to Include in Your CER:
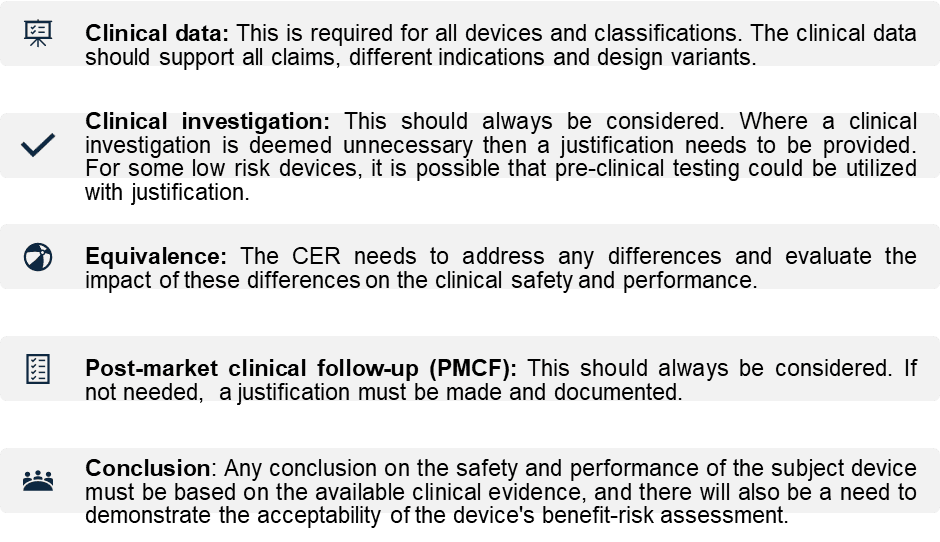
- Device Description and Intended Use: Include the regulatory history, classification, and intended purpose of your medical device or SaMD.
- Safety and Performance Claims: Ensure that the safety and performance of your device meet the requirements set out in the EU MDR.
- Clinical Background & State of the Art: Provide relevant context regarding the device’s clinical background and its position relative to other devices in the market.
- Clinical Evidence:
- Demonstrate equivalence to other devices if applicable.
- Summarise findings from systematic literature searches, clinical studies, and non-clinical testing.
- Provide evidence from post-market surveillance (PMS) and sales data.
- Include relevant usability data and risk management findings.
- Demonstrate equivalence to other devices if applicable.
- Benefit-Risk Assessment: Evaluate the benefit-risk profile based on available data and consider any post-market clinical follow-up (PMCF) requirements.
- Compliance with MDR Annex I Requirements: Conclude whether your device conforms with General Safety and Performance Requirements (GSPRs).
How Should a Clinical Evaluation Report Be Structured?
To ensure a comprehensive CER, your report should be structured in a way that demonstrates patient safety and device effectiveness. Below is a typical structure for a clinical evaluation report for medical devices and SaMD:
- Introduction: Brief overview of the device, intended use, and regulatory history.
- Clinical Evidence:
- Review of clinical data, including literature search results and comparison with equivalent devices.
- Summary of non-clinical testing and any usability studies.
- Review of clinical data, including literature search results and comparison with equivalent devices.
- Appraisal of Data: Assess the relevance and quality of the evidence.
- Benefit-Risk Assessment: Discuss the potential benefits and risks.
- Post-Market Surveillance: Highlight how post-market data is incorporated into the ongoing evaluation.
Conclusion: Final assessment of whether the device complies with EU MDR safety and performance standards.
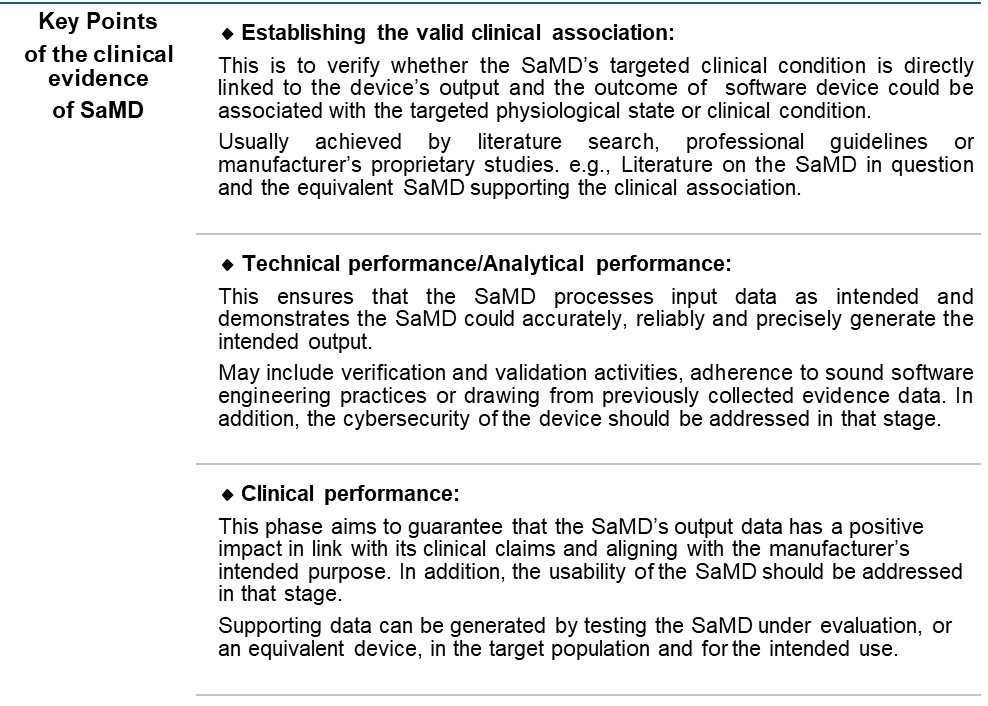
Clinical Evaluation for Software as a Medical Device (SaMD)
Software products such as diagnostic apps, monitoring tools, and AI-based algorithms fall under the broader category of Software as a Medical Device (SaMD). SaMD requires the same regulatory rigor as traditional medical devices under EU MDR.
Key differences when preparing the clinical evaluation report for SaMD include:
- Determining the classification of your SaMD.
- Establishing if the software requires a clinical evaluation based on its intended use.
Ensuring that the clinical evidence supports the safety and performance claims of the software, particularly if it involves diagnostic or therapeutic functionalities.
When Should You Update Your Clinical Evaluation?
The clinical evaluation process is not a one-time event; it must be an ongoing activity throughout the device lifecycle. After CE marking, you must continually update the clinical evaluation report based on:
- New clinical data from post-market use.
- Adverse event reports and complaints.
- Changes to regulatory requirements.
- Post-market clinical follow-up (PMCF) studies.
By keeping your clinical evaluation report up-to-date, you ensure that your device remains compliant with EU MDR and that the safety and effectiveness of your device are continually monitored.
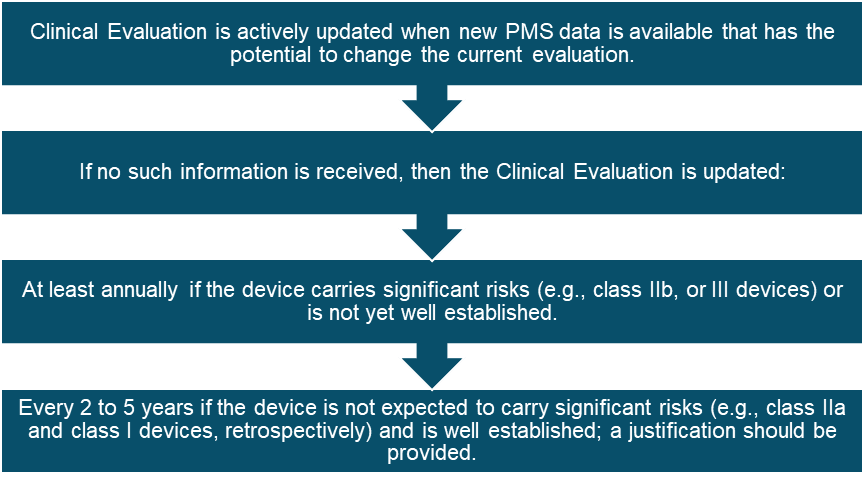
The Ongoing Process of Clinical Evaluation Under EU MDR
Creating an effective clinical evaluation report for medical devices and SaMD is just the beginning. The clinical evaluation process is ongoing, requiring regular updates and assessments throughout the device’s lifecycle. This ensures that your device continues to meet EU MDR requirements and remains safe and effective for its intended users.
Our team at LFH Regulatory can help you navigate the complexities of clinical evaluation reports and ensure compliance with the latest EU MDR guidelines. Contact us today at info@lfhregulatory.co.uk or call +44 (0) 1484 662575 for expert advice on your clinical evaluation needs.
FAQ’s for Creating Effective Clinical Evaluation Reports
What is a Clinical Evaluation Report (CER) for medical devices?
A CER is a detailed document that evaluates clinical evidence to prove a medical device or SaMD is safe, effective, and meets its intended use under EU MDR 2017/745. It combines pre-market and post-market data to support regulatory compliance.
Why do medical devices need a clinical evaluation?
Clinical evaluation is required to show that devices are safe for patients, perform as intended, and meet EU MDR standards. It is essential for CE marking and legal marketing in Europe.
What should be included in a Clinical Evaluation Report?
A complete CER must cover:
Device description and intended use
Safety and performance claims
Clinical background and state of the art
Evidence from studies, literature, and post-market data
Benefit-risk assessment
Compliance with EU MDR Annex I requirements
How do I structure a CER for regulatory submission?
A well-structured CER includes:
Introduction: device overview and regulatory context
Clinical evidence review: studies, literature, and equivalence
Appraisal of data: relevance and quality assessment
Benefit-risk assessment: evaluating safety vs. effectiveness
Post-market surveillance integration
Conclusion: confirmation of compliance with EU MDR
Does Software as a Medical Device (SaMD) require clinical evaluation?
Yes. SaMD, including apps, diagnostic tools, and AI algorithms, requires a clinical evaluation that demonstrates the software’s safety, performance, and regulatory compliance, similar to traditional medical devices.
How do I gather clinical evidence for a CER?
Evidence comes from:
Clinical trials and studies
Systematic literature searches
Post-market surveillance data and real-world use
Non-clinical testing
Usability and human factors studies
When should a Clinical Evaluation Report be updated?
CERs should be updated throughout the device lifecycle, whenever:
New clinical or post-market data emerges
Regulatory requirements change
PMCF (post-market clinical follow-up) studies are conducted
Device features are modified or updated
What is Post-Market Clinical Follow-Up (PMCF) in a CER?
PMCF involves collecting ongoing clinical data after the device is on the market. It helps identify safety issues, assess performance, and update the CER to maintain EU MDR compliance.
What happens if a CER is incomplete or outdated?
An incomplete CER can result in regulatory non-compliance, delays in CE marking, market access issues, or potential product recalls. Keeping the CER current ensures patient safety and legal compliance.
How can LFH Regulatory help with clinical evaluations?
LFH Regulatory provides expert support for planning, conducting, and documenting clinical evaluations for medical devices and SaMD. We ensure your CER meets EU MDR requirements and is ready for submission.
- Laura Friedl-Hirst
- Laura Friedl-Hirst
- Laura Friedl-Hirst
- Laura Friedl-Hirst
- Laura Friedl-Hirst
- Laura Friedl-Hirst
- Laura Friedl-Hirst
- Laura Friedl-Hirst
- Laura Friedl-Hirst
- Laura Friedl-Hirst
- Laura Friedl-Hirst
- Laura Friedl-Hirst
- Laura Friedl-Hirst
- Laura Friedl-Hirst
- Laura Friedl-Hirst
- Laura Friedl-Hirst
- Laura Friedl-Hirst
- Laura Friedl-Hirst
- Laura Friedl-Hirst
- Laura Friedl-Hirst
- Laura Friedl-Hirst
- Laura Friedl-Hirst