Mastering Medical Device Compliance: The 5 Crucial Stages of Regulatory Intelligence
In the rapidly evolving world of medical devices, staying compliant with regulatory intelligence can feel like a monumental task. The constant updates, changes, and complex requirements can be overwhelming. But don’t worry, we’re here to help. By following five fundamental stages, you can proactively manage regulatory compliance and stay ahead of the curve.
Let’s dive into these five essential stages and explore how they can help keep regulatory intelligence, and therefore compliance, at the forefront of your business strategy.
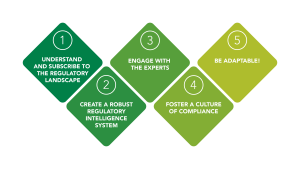
1. Understand and Subscribe to the Regulatory Landscape
The first step in mastering compliance is to understand the regulatory environment you’re working within. Familiarise yourself with the key regulatory bodies and agencies relevant to the regions where your medical devices are marketed. Whether you’re operating within the EU, UK, US, or any other jurisdiction, knowing the specific regulations that govern your device is crucial.
To ensure you’re always up-to-date, subscribe to newsletters, alerts, and updates from these regulatory bodies. Many organisations offer email subscriptions that provide timely information on new regulations, guidance documents, and compliance requirements. This will help you stay informed and act swiftly when changes arise.
2. Create a Robust Regulatory Intelligence System
A strong regulatory intelligence system is essential for streamlining compliance management. These systems allow you to:
- Monitor Regulatory Changes: Track updates in regulations, standards, and guidance documents relevant to your medical devices and the regions you operate in.
- Assess the Impact: Analyse how regulatory updates affect your products and processes. Implement necessary changes to remain compliant.
- Maintain Centralised Compliance Records: Store all compliance documents in one place, ensuring adherence to good documentation practices (GDP).
A successful regulatory intelligence system doesn’t stop at tracking updates; it also involves ongoing training for your team. Regular workshops and short training sessions help your staff stay informed on the latest regulatory requirements. This proactive approach ensures that your team is always ready to navigate the complexities of medical device compliance.
3. Engage with Regulatory Experts
Sometimes, navigating the complex regulatory landscape requires expert advice. Consult with regulatory experts and medical device consultancies that specialise in global regulations. Their insights can help you interpret complex regulations, manage compliance challenges, and navigate product development and approval processes more effectively.
Working with experts also ensures that you’re in the best position to make informed decisions that benefit both your compliance strategy and product development.
4. Foster a Culture of Compliance
Regulatory compliance shouldn’t just be a top-down directive, it needs to be part of the company culture. Encourage open discussions about compliance, and promote the importance of understanding regulatory intelligence at every level of the organisation. When every team member understands the role they play in compliance, it becomes a shared responsibility.
This culture of compliance not only helps with adhering to regulations but also creates an environment where questions are welcomed, and continuous improvement is encouraged.
5. Be Adaptable to Regulatory Changes
The regulatory landscape is constantly changing, and being adaptable is key to staying compliant. Regularly review your processes and systems to ensure they can handle evolving regulations. By being proactive, you’ll be better prepared to tackle compliance challenges as they arise.
An adaptable regulatory framework doesn’t just help ensure compliance; it also improves the safety and effectiveness of your medical devices, benefitting both healthcare providers and patients.
Need Help with Regulatory Intelligence?
Staying on top of the ever-changing regulatory landscape can be daunting, but you don’t have to do it alone. If you have any questions or need guidance on managing regulatory intelligence for your medical devices, get in touch with our expert consultants today. With deep knowledge of the global regulatory landscape, we can help you stay compliant, reduce risks, and achieve success.
Contact Us
Phone: +44 1484 662575
Email: info@lfhregulatory.co.uk
Or complete the ‘Contact Us’ form below to learn more about how we can support your regulatory needs.
FAQ’s for Regulatory Intelligence
What is regulatory intelligence in medical devices?
Regulatory intelligence is the process of gathering, monitoring, and analysing information about regulations, standards, and guidance that apply to medical devices in different regions. It helps businesses stay compliant and anticipate changes.
Why is regulatory intelligence important for medical device companies?
It ensures companies remain compliant with evolving laws, reduce risks of product delays or recalls, and maintain patient safety while bringing products to market efficiently.
How can companies stay updated with regulatory changes?
Businesses should subscribe to updates from regulatory bodies, set up a regulatory intelligence system, and engage with expert consultants who track changes across global markets
What are the five stages of regulatory intelligence?
The stages are:
Understand and subscribe to the regulatory landscape
Create a robust regulatory intelligence system
Engage with regulatory experts
Foster a culture of compliance
Be adaptable to regulatory changes
When should medical device companies seek expert regulatory advice?
Expert advice is valuable when interpreting complex regulations, entering new markets, or preparing for product development and approval processes.
- Laura Friedl-Hirst
- Laura Friedl-Hirst
- Laura Friedl-Hirst
- Laura Friedl-Hirst